Sunday, May 22, 2005
Dear Students:
Best of luck during Finals this year. The best bet is to really review those old Residencies and to complete the Study Guide!!! Remember to bring your completed Exam Study Guide Packet for 15 pts on your exam day.
http://www.studygs.net/ Check this out for Study Hints.
Best,
J. Gibney
Sample Final Residency Questions
Sample Final Residency Questions
____ 1. A gas-like mixture with no definite volume or shape that is made up of positively and negatively charged particles is a ____.
a. gas b. liquid c. plasma d. solid
____ 2. Matter in which the particles are free to move in all directions until they have spread evenly throughout their container is a ____.
a. gas b. liquid c. plasma d. solid
____ 3. Most matter ____ when heated.
a. condenses b. contracts c. expands d. solidifies
____ 4. The amount of energy needed to change a material from a solid to a liquid is called the heat of ____.
a. condensation b. rock n roll c. fusion d. vaporization
____ 5. The amount of energy needed to change a material from a liquid to a gas is called the heat of ____.
a. condensation b. Gibney magic c. fusion d. vaporization
____ 6. The idea that matter is made up of small particles that are in constant motion is ____.
a. Gibney’s principle b. heat of fusion c. Charles’s law d. the kinetic theory of matter
____ 7. A fluid’s resistance to flow is called ____.
a. viscosity b. fluid pressure c. buoyancy d. stickiness
____ 8. As the temperature of a gas increases, the volume of the gas will ____. Think of a balloon on a water bottle and you heat up the bottle.
a. decrease b. increase c. remain the same d. contract
____ 9. Fog is an example of a ____.
a. colloid b. compound c. solution d. substance
____ 10. Each inner energy level of an atom has a maximum number of ____ it can hold.
a. electrons b. neutrons c. quarks d. protons
____ 11. Dot diagrams are used to represent ____.
a. # of writeups you’ve gotten b. atomic mass c. isotopes d. outer level electrons (valence)
____ 12. Particles of matter that make up protons and neutrons are ____.
a. electrons b. isotopes c. quarks d. atoms
____ 13. Horizontal rows of the periodic table are called ____.
a. clusters b. families c. groups d. periods
____ 14. Atoms of the same element with different numbers of neutrons are called ____.
a. isotopes b. metals c. metalloids d. isomers
____ 15. A particle that moves around the nucleus is a(n) ____.
a. electron b. proton c. neutron d. quark
____ 16. A certain atom has 26 protons, 26 electrons, and 30 neutrons. Its mass number is ____.
a. 26 b. 30 c. 52 d. 56
____ 17. Elements that lie along the stair-step line of the periodic table are ____.
a. liquids b. metals c. metalloids d. radioactive
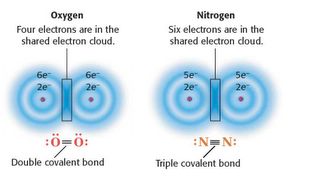
____ 18. A chemical bond that occurs when atoms share electrons is a(n) ____ bond.
a. covalent b. ionic c. booya d. polyatomic
____ 19. How many electrons are needed in the outer energy levels of most atoms for the atom to be chemically stable?
a. 2 b. 4 c. 6 d. 8
____ 20. How many hydrogen atoms are present in one molecule of ammonium acetate, NH4C2H3O2?
a. 4 b. 7 c. 11 d. 12
____ 21. How many potassium atoms (K) are in the following: 2K3PO4?
a. 6 b. 5 c. 24 d. booya
____ 22. Metals can be used as wire because they are ____.
a. alloys b. ductile c. metallic d. 100% chill
____ 23. In a solution, the substance that is being dissolved is the ____. Ex: the sugar in the iced-tea.
a. gas b. liquid c. solute d. solvent
____ 24. A solution that contains all of the solute it can hold at a given temperature is ____.
a. diluted b. saturated c. sweet d. unsaturated
____ 25. Increasing the surface area of a solid ____.
a. causes the solid to ionize b. has no effect on the rate of dissolving c. slows the rate of dissolving d. speeds the rate of dissolving
____ 26. The amount of solute that can be dissolved in a specific amount of solvent at a given temperature is its ____.
a. freezing point b. density c. dilution d. solubility
____ 27. Which of the following will speed up the dissolving of a solid solute in water?
a. Cool the solution. b. Freeze the solute. c. Watch it in the lab d. Stir the solution.
____ 28. Each substance on the left side of the arrow in a chemical equation is a ____.
a. catalyst b. coefficient c. product d. reactant
____ 29. If heat must be added to a chemical reaction for the reaction to take place, the reaction is ____.
a. balanced b. endothermic c. exothermic d. pretty good looking
____ 30. When one element replaces another element in a compound, the reaction is a ____ reaction.
a. Gibneysha reaction b. double–displacement c. single–displacement d. synthesis
____ 31. The breaking down of a substance into two or more simpler substances is ____.
a. decomposition b. displacement c. a catalyst d. synthesis
____ 32. Each substance to the right of the arrow in a chemical equation is a(n) ____.
a. Gibney buck b. inhibitor c. reactant d. product
____ 33. A chemical reaction in which heat energy is released is ____.
a. endothermic b. exothermic c. flammable d. a formula
____ 34. According to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
a. There is no relationship. b. The mass of the products is greater. c. The mass of the reactants is greater. d. The masses are equal.
____ 35. A(n) ____ is a substance that produces H+ ions in a water solution. (Has pH less than 7)
a. acid b. base c. salt d. alcohol
____ 36. A(n) ____ is a substance that produces OH- ions in a solution. (Has pH greater than 7)
a. acid b. base c. salt d. alcohol
____ 37. ____ measures how acidic or basic a substance is.
a. An ester b. A base c. pH scale d. The hydronium ion
____ 38. Coffee has a pH of about 5. Coffee is ____.
a. extremely cool b. extremely basic c. somewhat acidic d. somewhat basic
____ 39. Dontrielle wants to study the effect of light on the growth of bean seedlings. How should she set up her experiment?
a. She should grow ten bean seedlings in a lighted area. b. She should grow ten bean seedlings in a dark area. c. She should grow five bean seedlings in a lighted area and eat the five other beans d. She should grow five bean seedlings in a lighted area and five bean seedlings in a dark area.
____ 40. A housepainter uses paint thinner to remove paint from her hands. Paint thinner is the ____.
a. acid b. base c. solute d. solvent
____ 41. The most common state of matter in the universe is ____.
a. gas b. liquid c. plasma d. solid
____ 42. How many electrons does a carbon atom have in its outer energy level?
a. 2 b. 4 c. 6 d. 8
____ 43. In a chemical equation, the symbol that means dissolved in water is ____.
a. (aq) b. (s) c. (dw) d. (I)
____ 44. Numbers that precede symbols and formulas in a chemical equation are ____.
a. catalysts b. coefficients c. superscripts d. subscripts
____ 45. ____ of a solution refers to the ease with which an acid or base forms ions in solution.
a. Acidity b. Concentration c. pH d. Strength
____ 46. Data from the U.S. Census shows that people who have No High School Diploma earn $15,570 a year, people with a High School Diploma earn $22,481 a year and people with a Bachelor’s Degree earn .
a. $0 a year b. $40,347 a year c. cost of a cold drink d. 2 Benjamins a year
____ 47. The charge of an electron is
a. -2. b. -1. c. 0. d. +1.
____ 48. Oxygen’s atomic number is 8. This means that an oxygen atom has
a. eight neutrons in its nucleus. b. a total of eight protons and neutrons. c. eight protons in its nucleus. d. a total of eight neutrons and electrons.
____ 49. An atom’s mass number equals the number of
a. protons plus the number of electrons. b. protons plus the number of neutrons. c. protons. d. neutrons.
____ 50. The heavier a particle, the _____ it moves.
a. slower b. faster c. less d. more
____ 51. The change of a substance from a solid directly to a gas is called
a. condensation. b. evaporation. c. melting. d. sublimation.
____ 52. Each molecule of table sugar, C12H22O11, contains
a. 0 atoms of carbon. b. 1 atom of carbon. c. 6 atoms of carbon. d. 12 atoms of carbon.
____ 53. Often atoms join so that each atom will have
a. an even number of electrons. b. an outermost energy level that is full of electrons. c. an equal number of protons and electrons. d. more electrons than either protons or neutrons.
____ 54. A mixture that separates into different layers when you stop stirring it is
a. a colloid. b. a suspension. c. a solution. d. an emulsion.
____ 55. Fusion occurs when nuclei
a. split. b. combine. c. mutate. d. gain energy.
Essay: 10 points each
56. Choose One:
A) Explain how a hot-air balloon works using Charles’s law.
B) What will happen to the size of a balloon when it is placed in a freezer? Explain.
57. Compare the masses of protons, electrons, and neutrons. Which are the lightest?
58. What is the name of each of the following elements, and classify it as a metal, a nonmetal, or a metalloid: Na, Ba, Ca, La, Ti, Al, As, At, Ar.
Phys Final Exam Spring 2005
Answer Section
MULTIPLE CHOICE
1. C
2. A
3. C
4. C
5. D
6. D
7. A
8. B
9. A
10. A
11. D
12. C
13. D
14. A
15. A
16. D
17. C
18. A
19. D
20. B
21. A
22. B
23. C
24. B
25. D
26. D
27. D
28. D
29. B
30. C
31. A
32. D
33. B
34. D
35. A
36. B
37. C
38. C
39. D
40. D
41. C
42. B
43. A
44. B
45. D
46. B
47. B
48. C
49. B
50. A
51. D
52. D
53. B
54. B
55. B
SHORT ANSWER
56. Charles’s law—as air is heated, it expands;
It shrinks; as the temperature of air decreases, the volume of the air decreases.
57. The mass of a proton and a neutron are about the same, and the electron is about 1,836 times smaller.
58. Na, sodium, metal; Ba, barium, metal; Ca, calcium, metal; La, lanthanum, metal; Ti, titanium, metal; Al, aluminum, metal; As, arsenic, metalloid; At, astatine, metalloid; Ar, argon, nonmetal
____ 1. A gas-like mixture with no definite volume or shape that is made up of positively and negatively charged particles is a ____.
a. gas b. liquid c. plasma d. solid
____ 2. Matter in which the particles are free to move in all directions until they have spread evenly throughout their container is a ____.
a. gas b. liquid c. plasma d. solid
____ 3. Most matter ____ when heated.
a. condenses b. contracts c. expands d. solidifies
____ 4. The amount of energy needed to change a material from a solid to a liquid is called the heat of ____.
a. condensation b. rock n roll c. fusion d. vaporization
____ 5. The amount of energy needed to change a material from a liquid to a gas is called the heat of ____.
a. condensation b. Gibney magic c. fusion d. vaporization
____ 6. The idea that matter is made up of small particles that are in constant motion is ____.
a. Gibney’s principle b. heat of fusion c. Charles’s law d. the kinetic theory of matter
____ 7. A fluid’s resistance to flow is called ____.
a. viscosity b. fluid pressure c. buoyancy d. stickiness
____ 8. As the temperature of a gas increases, the volume of the gas will ____. Think of a balloon on a water bottle and you heat up the bottle.
a. decrease b. increase c. remain the same d. contract
____ 9. Fog is an example of a ____.
a. colloid b. compound c. solution d. substance
____ 10. Each inner energy level of an atom has a maximum number of ____ it can hold.
a. electrons b. neutrons c. quarks d. protons
____ 11. Dot diagrams are used to represent ____.
a. # of writeups you’ve gotten b. atomic mass c. isotopes d. outer level electrons (valence)
____ 12. Particles of matter that make up protons and neutrons are ____.
a. electrons b. isotopes c. quarks d. atoms
____ 13. Horizontal rows of the periodic table are called ____.
a. clusters b. families c. groups d. periods
____ 14. Atoms of the same element with different numbers of neutrons are called ____.
a. isotopes b. metals c. metalloids d. isomers
____ 15. A particle that moves around the nucleus is a(n) ____.
a. electron b. proton c. neutron d. quark
____ 16. A certain atom has 26 protons, 26 electrons, and 30 neutrons. Its mass number is ____.
a. 26 b. 30 c. 52 d. 56
____ 17. Elements that lie along the stair-step line of the periodic table are ____.
a. liquids b. metals c. metalloids d. radioactive
____ 18. A chemical bond that occurs when atoms share electrons is a(n) ____ bond.
a. covalent b. ionic c. booya d. polyatomic
____ 19. How many electrons are needed in the outer energy levels of most atoms for the atom to be chemically stable?
a. 2 b. 4 c. 6 d. 8
____ 20. How many hydrogen atoms are present in one molecule of ammonium acetate, NH4C2H3O2?
a. 4 b. 7 c. 11 d. 12
____ 21. How many potassium atoms (K) are in the following: 2K3PO4?
a. 6 b. 5 c. 24 d. booya
____ 22. Metals can be used as wire because they are ____.
a. alloys b. ductile c. metallic d. 100% chill
____ 23. In a solution, the substance that is being dissolved is the ____. Ex: the sugar in the iced-tea.
a. gas b. liquid c. solute d. solvent
____ 24. A solution that contains all of the solute it can hold at a given temperature is ____.
a. diluted b. saturated c. sweet d. unsaturated
____ 25. Increasing the surface area of a solid ____.
a. causes the solid to ionize b. has no effect on the rate of dissolving c. slows the rate of dissolving d. speeds the rate of dissolving
____ 26. The amount of solute that can be dissolved in a specific amount of solvent at a given temperature is its ____.
a. freezing point b. density c. dilution d. solubility
____ 27. Which of the following will speed up the dissolving of a solid solute in water?
a. Cool the solution. b. Freeze the solute. c. Watch it in the lab d. Stir the solution.
____ 28. Each substance on the left side of the arrow in a chemical equation is a ____.
a. catalyst b. coefficient c. product d. reactant
____ 29. If heat must be added to a chemical reaction for the reaction to take place, the reaction is ____.
a. balanced b. endothermic c. exothermic d. pretty good looking
____ 30. When one element replaces another element in a compound, the reaction is a ____ reaction.
a. Gibneysha reaction b. double–displacement c. single–displacement d. synthesis
____ 31. The breaking down of a substance into two or more simpler substances is ____.
a. decomposition b. displacement c. a catalyst d. synthesis
____ 32. Each substance to the right of the arrow in a chemical equation is a(n) ____.
a. Gibney buck b. inhibitor c. reactant d. product
____ 33. A chemical reaction in which heat energy is released is ____.
a. endothermic b. exothermic c. flammable d. a formula
____ 34. According to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
a. There is no relationship. b. The mass of the products is greater. c. The mass of the reactants is greater. d. The masses are equal.
____ 35. A(n) ____ is a substance that produces H+ ions in a water solution. (Has pH less than 7)
a. acid b. base c. salt d. alcohol
____ 36. A(n) ____ is a substance that produces OH- ions in a solution. (Has pH greater than 7)
a. acid b. base c. salt d. alcohol
____ 37. ____ measures how acidic or basic a substance is.
a. An ester b. A base c. pH scale d. The hydronium ion
____ 38. Coffee has a pH of about 5. Coffee is ____.
a. extremely cool b. extremely basic c. somewhat acidic d. somewhat basic
____ 39. Dontrielle wants to study the effect of light on the growth of bean seedlings. How should she set up her experiment?
a. She should grow ten bean seedlings in a lighted area. b. She should grow ten bean seedlings in a dark area. c. She should grow five bean seedlings in a lighted area and eat the five other beans d. She should grow five bean seedlings in a lighted area and five bean seedlings in a dark area.
____ 40. A housepainter uses paint thinner to remove paint from her hands. Paint thinner is the ____.
a. acid b. base c. solute d. solvent
____ 41. The most common state of matter in the universe is ____.
a. gas b. liquid c. plasma d. solid
____ 42. How many electrons does a carbon atom have in its outer energy level?
a. 2 b. 4 c. 6 d. 8
____ 43. In a chemical equation, the symbol that means dissolved in water is ____.
a. (aq) b. (s) c. (dw) d. (I)
____ 44. Numbers that precede symbols and formulas in a chemical equation are ____.
a. catalysts b. coefficients c. superscripts d. subscripts
____ 45. ____ of a solution refers to the ease with which an acid or base forms ions in solution.
a. Acidity b. Concentration c. pH d. Strength
____ 46. Data from the U.S. Census shows that people who have No High School Diploma earn $15,570 a year, people with a High School Diploma earn $22,481 a year and people with a Bachelor’s Degree earn .
a. $0 a year b. $40,347 a year c. cost of a cold drink d. 2 Benjamins a year
____ 47. The charge of an electron is
a. -2. b. -1. c. 0. d. +1.
____ 48. Oxygen’s atomic number is 8. This means that an oxygen atom has
a. eight neutrons in its nucleus. b. a total of eight protons and neutrons. c. eight protons in its nucleus. d. a total of eight neutrons and electrons.
____ 49. An atom’s mass number equals the number of
a. protons plus the number of electrons. b. protons plus the number of neutrons. c. protons. d. neutrons.
____ 50. The heavier a particle, the _____ it moves.
a. slower b. faster c. less d. more
____ 51. The change of a substance from a solid directly to a gas is called
a. condensation. b. evaporation. c. melting. d. sublimation.
____ 52. Each molecule of table sugar, C12H22O11, contains
a. 0 atoms of carbon. b. 1 atom of carbon. c. 6 atoms of carbon. d. 12 atoms of carbon.
____ 53. Often atoms join so that each atom will have
a. an even number of electrons. b. an outermost energy level that is full of electrons. c. an equal number of protons and electrons. d. more electrons than either protons or neutrons.
____ 54. A mixture that separates into different layers when you stop stirring it is
a. a colloid. b. a suspension. c. a solution. d. an emulsion.
____ 55. Fusion occurs when nuclei
a. split. b. combine. c. mutate. d. gain energy.
Essay: 10 points each
56. Choose One:
A) Explain how a hot-air balloon works using Charles’s law.
B) What will happen to the size of a balloon when it is placed in a freezer? Explain.
57. Compare the masses of protons, electrons, and neutrons. Which are the lightest?
58. What is the name of each of the following elements, and classify it as a metal, a nonmetal, or a metalloid: Na, Ba, Ca, La, Ti, Al, As, At, Ar.
Phys Final Exam Spring 2005
Answer Section
MULTIPLE CHOICE
1. C
2. A
3. C
4. C
5. D
6. D
7. A
8. B
9. A
10. A
11. D
12. C
13. D
14. A
15. A
16. D
17. C
18. A
19. D
20. B
21. A
22. B
23. C
24. B
25. D
26. D
27. D
28. D
29. B
30. C
31. A
32. D
33. B
34. D
35. A
36. B
37. C
38. C
39. D
40. D
41. C
42. B
43. A
44. B
45. D
46. B
47. B
48. C
49. B
50. A
51. D
52. D
53. B
54. B
55. B
SHORT ANSWER
56. Charles’s law—as air is heated, it expands;
It shrinks; as the temperature of air decreases, the volume of the air decreases.
57. The mass of a proton and a neutron are about the same, and the electron is about 1,836 times smaller.
58. Na, sodium, metal; Ba, barium, metal; Ca, calcium, metal; La, lanthanum, metal; Ti, titanium, metal; Al, aluminum, metal; As, arsenic, metalloid; At, astatine, metalloid; Ar, argon, nonmetal
Sunday, May 15, 2005
Webquest 5-16
We just watched a short video about fireworks. Now you are going to embark on an exciting journey through the chemistry of fireworks.
Kaboom! Oooh! Aahh! The golden sparkles explode and float down the darkened sky, thrilling everyone watching below. Every Fourth of July, millions of Americans go to local parks to watch exciting fireworks presentations. Fireworks have been a familiar part of celebrations for centuries. For most of that time, the designing of fireworks was a craft. Only recently have people begun to try and understand the science involved in creating the spectacular fireworks displays we all enjoy. What are the component parts of fireworks? What chemical compounds cause fireworks to explode? What chemical compounds are responsible for the colors of fireworks?
Your job in this WebQuest is to discover the component parts of fireworks, and to identify the chemical compounds that are responsible for the brilliant colors that light up the sky as fireworks explode. You will explore the history of fireworks and find out when the first fireworks were invented. You will learn about firework design and how fireworks are built. You will also find out what chemical compounds are responsible for the colors seen in fireworks. Finally, you will answer a set of questions about fireworks to demonstrate what you have learned about the chemistry of fireworks.
SURVEY LINK FOR YOUR ANSWERS!!!
WEBSITES
A History of Fireworks. At this site, you can learn about the history of fireworks.
Where did fireworks begin?
Professional Colors. Discussed are some of the reasons some fireworks are brighter then others. You will also find a chart of chemicals that will produce certain colors of fireworks.
Lights and Colours. Go to this site to see what chemicals create the colors of firesworks. Before the 19th century, only the colors white, yellow, and orange were possible in fireworks. When did the colors red, green, blue, and purple become possible in fireworks?
How Fireworks are Made. At this site you can find out what chemical compounds create the colors of modern fireworks.
NOVA Online: Kaboom! Go to this site for a diagram of the parts of a modern firework. Each part of the diagram has an active label. Click on each label to learn more about that part of the firework.
The Chemistry of Fireworks. Visit this site to learn more about the chemical reactions in fireworks. Find out what two types of binders are used in fireworks today.
Kaboom! Oooh! Aahh! The golden sparkles explode and float down the darkened sky, thrilling everyone watching below. Every Fourth of July, millions of Americans go to local parks to watch exciting fireworks presentations. Fireworks have been a familiar part of celebrations for centuries. For most of that time, the designing of fireworks was a craft. Only recently have people begun to try and understand the science involved in creating the spectacular fireworks displays we all enjoy. What are the component parts of fireworks? What chemical compounds cause fireworks to explode? What chemical compounds are responsible for the colors of fireworks?
Your job in this WebQuest is to discover the component parts of fireworks, and to identify the chemical compounds that are responsible for the brilliant colors that light up the sky as fireworks explode. You will explore the history of fireworks and find out when the first fireworks were invented. You will learn about firework design and how fireworks are built. You will also find out what chemical compounds are responsible for the colors seen in fireworks. Finally, you will answer a set of questions about fireworks to demonstrate what you have learned about the chemistry of fireworks.
SURVEY LINK FOR YOUR ANSWERS!!!
WEBSITES
A History of Fireworks. At this site, you can learn about the history of fireworks.
Where did fireworks begin?
Professional Colors. Discussed are some of the reasons some fireworks are brighter then others. You will also find a chart of chemicals that will produce certain colors of fireworks.
Lights and Colours. Go to this site to see what chemicals create the colors of firesworks. Before the 19th century, only the colors white, yellow, and orange were possible in fireworks. When did the colors red, green, blue, and purple become possible in fireworks?
How Fireworks are Made. At this site you can find out what chemical compounds create the colors of modern fireworks.
NOVA Online: Kaboom! Go to this site for a diagram of the parts of a modern firework. Each part of the diagram has an active label. Click on each label to learn more about that part of the firework.
The Chemistry of Fireworks. Visit this site to learn more about the chemical reactions in fireworks. Find out what two types of binders are used in fireworks today.
Monday, May 09, 2005
Sunday, May 01, 2005
Sunday, April 24, 2005
2nd Semester Projects
Please remember that your 2nd Semester Project is due 4/28. View your project not as a stinky thing to do, but as a training exercise for College. Trust me… the ability to perform research and present information is one of the biggest skills needed for the big show - College. Give me your best and be proud of your work!!!
Top 5 Project Tips:
5. Don’t Plagiarize!!! That means no copying a report off of the internet.
4. Be Creative – Add your own unique twist to your project
3. Turn your Project in on Time. You will lose 25 pts per day it is late.
2. Ask Mr. G questions
1. Try your best… that’s all I can ask!
Some helpful project links:
Steve Spangler Science Experiments
Home Experiments
Bizzare Stuff You Can Make in Your Kitchen
How Stuff Works – Look here for info on Bombs
Yucca Mountain
Top 5 Project Tips:
5. Don’t Plagiarize!!! That means no copying a report off of the internet.
4. Be Creative – Add your own unique twist to your project
3. Turn your Project in on Time. You will lose 25 pts per day it is late.
2. Ask Mr. G questions
1. Try your best… that’s all I can ask!
Some helpful project links:
Steve Spangler Science Experiments
Home Experiments
Bizzare Stuff You Can Make in Your Kitchen
How Stuff Works – Look here for info on Bombs
Yucca Mountain
Sunday, April 17, 2005
Tuesday, April 12, 2005
THE ART OF NEON WEBQUEST!!!! 4-13
Your job in this WebQuest is to explore the chemistry behind neon signs, and learn how the different colors of these signs are made. You will discover exactly how the noble gases are inserted into glass tubing, and how the gases are made to glow. You will also learn how different colors of neon signs are created. You will find out how neon glass tubing can be bent and how neon artists create their works of art. Then you will answer a set of questions about neon signs. If time permits you might design your own Neon art!
Access the online activity here!
Resources for the Online Activity:
WebElements – The Periodic Table on the WWW. Visit this site for information on the noble gases. You can click on any element in this periodic table to read about that element and its properties. Click on any of the noble gases to find out about that element.
Krypton Neon – Everything you ever wanted to know about Neon. Go to this site to find the answers to questions you might have about neon and neon lights. This site is intended more for neon artists than chemistry students.
Neon Knights: Part 1: An Exploration of Neon. At this site you can find out about the discovery of neon and how neon signs operate. Scroll down and click on how are artists using neon to learn more about neon as art.
Neon Colors. Visit this site to see many of the possible colors found in neon signs. Notice that colors listed are made with just three elements.
Just Neon’s FAQ Page. Go to this site to find out more about neon signs. You can learn exactly how a noble gas is introduced and held inside a glass tube at this site.
Neon: A Brief History of Signs. At this site you can find a history of neon’s discovery and its use in neon signs. You can find out here how the glass tubing used in neon signs is made.
The History of Neon Signs. Visit this site to learn about the inventor of the first neon lamp. You can find out how neon signs are made here, and about what colors are produced by using the elements argon and mercury.
Ray Kolty’s Neon FAQ. Go to this site for more information about how neon tubes light up, how neon tubes are made, and how the colors of neon tubes are created.
Museum of Neon Art. At this site you can see how artists are using neon tubing in artworks. Scroll down and click on web gallery to see some neon art pieces presently on exhibit at this museum.
Neon Artworks. Visit this site to see all kinds of uses for neon signs as art. Click on free standing sculptures to see some wonderful examples of neon art.
GlassLight Gallery. Go to this site to see neon art and neon sculpture. Scroll down and click on views of the luminous sculpture. On the menu at the left side of the screen, click on jellyfish to see an amazing array of neon sculptures.
Extra Sites to Check out:
Easy Science Experiments
Current Events
Access the online activity here!
Resources for the Online Activity:
WebElements – The Periodic Table on the WWW. Visit this site for information on the noble gases. You can click on any element in this periodic table to read about that element and its properties. Click on any of the noble gases to find out about that element.
Krypton Neon – Everything you ever wanted to know about Neon. Go to this site to find the answers to questions you might have about neon and neon lights. This site is intended more for neon artists than chemistry students.
Neon Knights: Part 1: An Exploration of Neon. At this site you can find out about the discovery of neon and how neon signs operate. Scroll down and click on how are artists using neon to learn more about neon as art.
Neon Colors. Visit this site to see many of the possible colors found in neon signs. Notice that colors listed are made with just three elements.
Just Neon’s FAQ Page. Go to this site to find out more about neon signs. You can learn exactly how a noble gas is introduced and held inside a glass tube at this site.
Neon: A Brief History of Signs. At this site you can find a history of neon’s discovery and its use in neon signs. You can find out here how the glass tubing used in neon signs is made.
The History of Neon Signs. Visit this site to learn about the inventor of the first neon lamp. You can find out how neon signs are made here, and about what colors are produced by using the elements argon and mercury.
Ray Kolty’s Neon FAQ. Go to this site for more information about how neon tubes light up, how neon tubes are made, and how the colors of neon tubes are created.
Museum of Neon Art. At this site you can see how artists are using neon tubing in artworks. Scroll down and click on web gallery to see some neon art pieces presently on exhibit at this museum.
Neon Artworks. Visit this site to see all kinds of uses for neon signs as art. Click on free standing sculptures to see some wonderful examples of neon art.
GlassLight Gallery. Go to this site to see neon art and neon sculpture. Scroll down and click on views of the luminous sculpture. On the menu at the left side of the screen, click on jellyfish to see an amazing array of neon sculptures.
Extra Sites to Check out:
Easy Science Experiments
Current Events